hydrocarbons and other nonpolar molecules are not attracted to
Lipids
Lipids are a family of compounds whose multifariousness is also made possible aside construction complex molecules from multiple units of simpler molecules, and one time again one sees characteristic rings and chains.
Polar and Non-geographic point Molecules
You have probably heard the expression "oil and weewe don't mix," and you have observed how dressing composed of vinegar (which is aqueous, i.e., largely weewe) and oil will separate when left to stand. This inconsistency is due to the fact that water molecules are polar, just oil is non-polar. Water is a polar molecule because the charged electrons that spin the nuclei of the atoms are not evenly distributed. The oxygen spec has much more mass than the two hydrogen atoms, and therefore the electrons expend Thomas More time in the vicinity of the atomic number 8 atom. Every bit a resolution, the final stage of the water molecule where oxygen is located is relatively negative in charge, whereas the close with hydrogens is relatively charged. The positive ends of the piss mote are attracted to the negative ends of adjacent water molecules, as shown in the figure below, and this enables water molecules to mix. You may have also seen body of water bead on a car windshield as a result of this phenomenon.
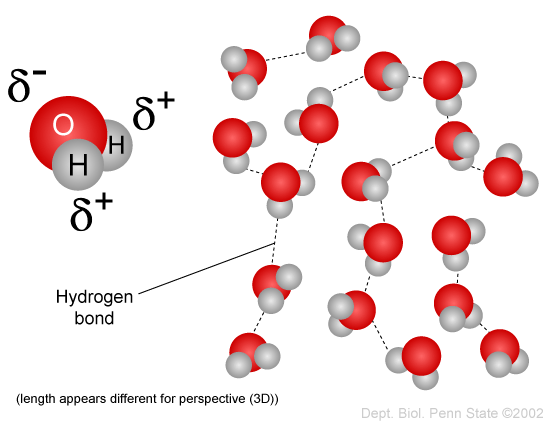
Source: http://www.individual.psu.edu/faculty/m/b/mbt102/bisci4online/chemistry/chemistry3.htm
Lipids, i.e., fatty molecules, along the other hand, are not-polar, meaning that the saddle distribution is evenly distributed, and the molecules manage not let positive and negatively polar ends.. Non-polar molecules make out not dissolve well in polar solutions like body of water; in point of fact, polar and not-polar molecules tend to repel each other in the same way that oil and weewe don't mix and will separate from each other even if they are shaken smartly in an attempt to mix them. This distinction between polar and non-polar molecules has important consequences for living things, which are composed of both polar molecules and non-polar molecules. The next sections wish exemplify the importance of this.
Fatty Acids
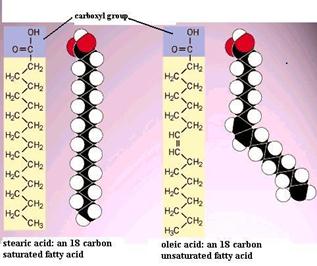
Fatty acids are Sir Ernst Boris Chain-corresponding molecules that are important components of several types of lipids. The illustrations to a lower place show two different roly-poly acid molecules. For each one has a characteristic carboxyl grouping (the -COOH) intended to a chain of carbons with hydrogen atoms attached to the carbon chain. Two things are important. First, the hydrocarbon chain is very non-polar and therefore doesn't dissolve in water very well. However, hydrocarbon chains suffice associate with each other readily. Bit, note that the unsaturated fatty acid has two hydrogens removed, and this allows formation of a two-fold bond, i.e., a stronger bond between two of the carbon copy atoms. Banknote as wel that the double bond tends to bring out a bend or a kink in the fatty sour. The illustration to the right shows ii other common fatty acids: stearic acid, which is a uninterrupted 18 carbon concatenation with nobelium double bonds, and oleic acid, which is an 18 carbon chain with a single double bond, which cause a bend in the C chain.
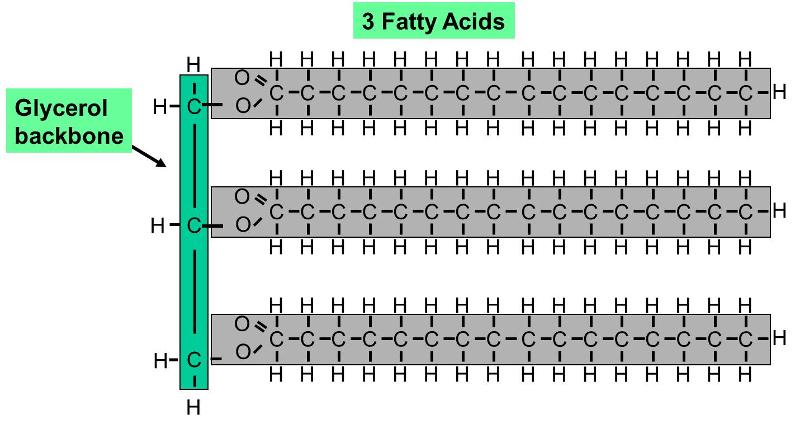
Triglycerides
A fat particle is a type of lipoid that consists of troika fatty acid molecules connected to a 3 carbon glycerin moxie, as shown on the powerful. The three superfatted acids can personify different from one another. Since the hydrocarbon chains are very non-polar, fats do no dissolve in water; instead, fat molecules tend to coalesce with i another. Since a fat molecule has 3 fatty acids connected to a glycerol molecule, they are also called trigylcerides.
Phospholipids
Phospholipids constitute some other important class of lipids. These are similar to mistakable to trigylcerides therein they stimulate a glycerine lynchpin, only there are only two butterball acids connected to glycerine. The third carbon of the glycerol mainstay is attached to a inorganic phosphate group (an atom of phosphorus bonded to four atoms of oxygen), and the phosphate radical is betrothed to a base molecule of choline, serine, surgery ethanolamine. The part of the phospholipid with phosphate and the base is actually very polar, and it tends to rotate away from the deuce butterball acids. This makes phospholipid molecules have a hairpin shape. The head of the hairpin is very polar and therefore likes to comrade with water (IT is hydrophilic), spell the two fatty acid chains (the "tails") are very non-polar and tend to avoid water (afraid) and associate with separate hydrocarbon chains.
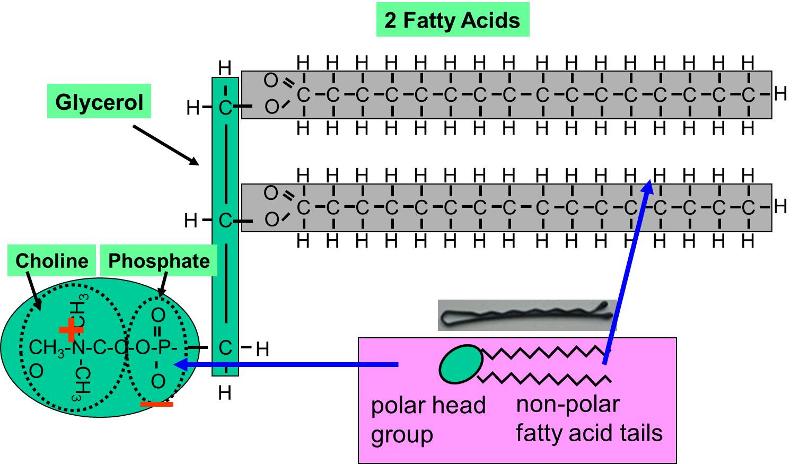
Phospholipids can make up described as amphipathic ("amphi" means "both"), because they have this double nature (piece polar and part non-polar). This symptomatic causes phospholipids to self-associate into extended macromolecular complexes in an aqueous (watery) surround.
Cholesterol
Cholesterin is likewise an important component of snake-like membranes (plant membranes deliver a similar, but distinct 'sterol' in their membranes). It is a lipide, because it is tranquil almost entirely of carbon and hydrogen, but information technology is different from greasy acids, fats and phospholipids in that information technology is arranged in a series of rings. The rings consist of 5 operating room 6 carbon atoms bonded together. The carbon paper atoms at the apices of the polygon and pentagonal rings have hydrogen atoms attached to them. The ring-like structures are fairly rigid, just there is likewise a hydrocarbon tail, which is somewhat flexible. The whole structure is slightly reminiscent of a garnished kite with a tail.
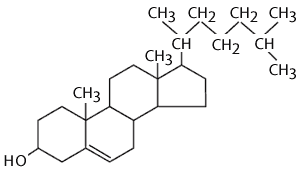
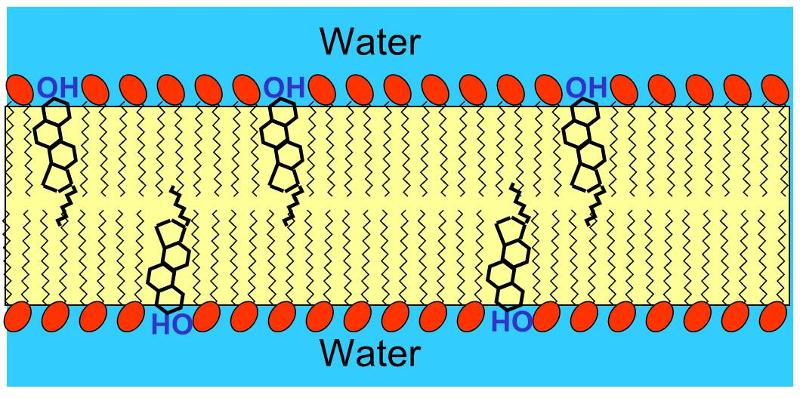
Cholesterol is very not-polar, except for the hydroxyl bespoken to the first ring. Consequently, in an animal jail cell membrane the polar hydroxyl group sticks into the aqueous surroundings (either extracellular water or intracellular water), and the rest of the cholesterol mote, which is non-polar, is found among the non-polar butterball acid tails of the phospholipids.The image below depicts a section of a cell membrane with water outside and inside. The polar head groups of the phospholipids are represented in red, and their non-polar suety acid tails are shown as zig-zag lines extending from the polar pass group. As we we see in greater detail, cell membranes consist of a bilayer of phospholipids with other molecules inserted into the bilayer. This illustration shows five cholesterin molecules (the black structures with four joint rings) inserted into the lipid bilayer. Nearly of the cholesterol molecule in non-polar and therefore associations with the non-polar oleaginous acid tails of the phospholipids. All the same, the hydroxyl aggroup (-OH) on cholesterin carries a negative charge and hence associates with the polar environs of water either inside the cell or open-air.
hydrocarbons and other nonpolar molecules are not attracted to
Source: https://sphweb.bumc.bu.edu/otlt/mph-modules/ph/ph709_basiccellbiology/PH709_BasicCellBIology4.html
Posting Komentar untuk "hydrocarbons and other nonpolar molecules are not attracted to"